It seems to be the latest trend amongst the Regenerative Ag folks to stop using seed-applied fungicides for fear of what they might be doing to the soil ecology, particularly mycorrhizae —my agronomy consulting is dba Pinnacle Crop Tech). Should we be avoiding seed-applied fungicides? It’s a complex, nuanced topic—so let’s have a look at what the evidence tells us.
South Dakota Cropland Soil: Corn, Soybean, & Oats
A recent, top-notch study[i] (Cameron et al.) delved into it. The USDA-ARS scientists isolated four mycorrhizae species from cropland field soil near Brookings, SD—unlike many previous studies that often only looked at a single mycorrhizal species, and perhaps not even an important one for field crops. The ARS team used three soybean varieties, three corn hybrids, and two oat varieties (soybean and oat varieties with higher mycorrhizal propensities were used). Oats was used as a proxy for wheat because of its stronger mycorrhizal associations (making it easier to study). Corn seed was treated with Cruiser Extreme, Stamina, or Trilex fungicide, or nothing. Soybeans were treated with CruiserMaxx Advanced, Evergol Energy SB, Vibrance, or nothing. Oats were treated with Raxil MD, Stamina F3 Cereals, Evergol Energy, or nothing. All were at labelled rates, and all of them contained at least one systemic fungicide ingredient.
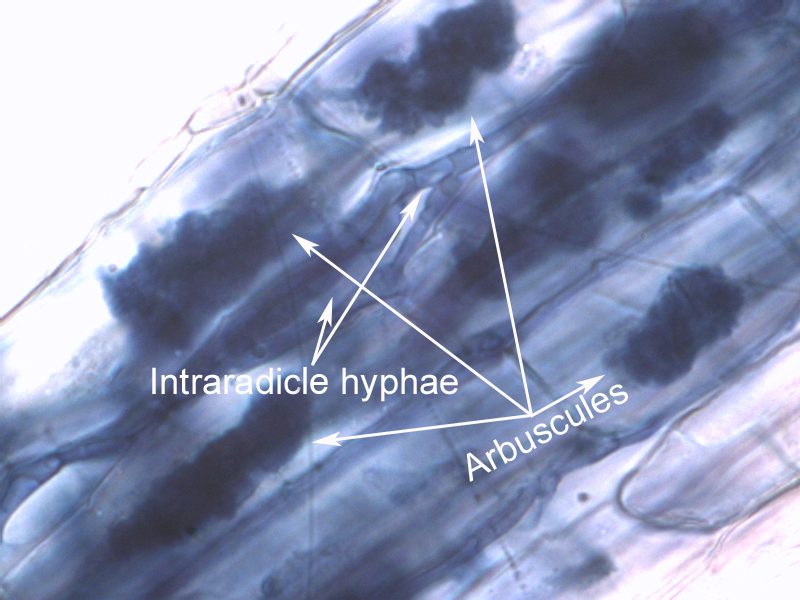
The crops were planted in greenhouse pots, in soil inoculated with the four mycorrhizae species isolated from Brookings, SD cropland. (The mycorrhizae were propagated on bahia grass culture, which was chopped up to inoculate the sterile media in the pots.) Phosphorous (P) levels were kept low, but other nutrients supplied. Corn was harvested 33 days after emergence: oats & soybean at 38 days. Roots were analyzed extensively (100 locations on the roots for each plant) under the microscope to determine mycorrhizal colonization levels (arbuscular only was counted, as well as total colonization which includes arbuscles, vesicles, and hyphae). Above ground plant material was weighed and analyzed for nutrient concentration.
For corn, none of the treatments caused a significant decrease in mycorrhizal colonization compared to the untreated control. There were some minor differences among them, with Cruiser Extreme significantly suppressing mycorrhizae compared to the other treatments (significant at P < 0.05). However, corn hybrid genetics resulted in greater differences in mycorrhizal colonization than between the seed treatments. Also, the corn treated with Stamina & Trilex tended to have higher arbuscular mycorrhizal (AM) levels than the control, although not significant at P < 0.05. Not much difference showed up in plant biomass or nutrient concentrations; no significant differences in P concentration were observed, although Cruiser Extreme tended to be slightly lower.
For soybean, once again, none of the treatments caused a significant change in mycorrhizal levels compared to control. Although variety did affect colonization. None of the varieties with any of the seed treatments had significantly lower P levels than the control; however, for one variety, CruiserMaxx and Evergol Energy resulted in higher P concentrations (indicating mycorrhizae may have been stimulated, not suppressed). Biomass wasn’t affected by treatment (these greenhouse pots were well-drained, and warm, so seedling disease wasn’t much of an issue; pathogen levels probably weren’t all that high since the soil was created with sterile ingredients plus the bahia grass roots).
For oats, treatments with Raxil MD had significantly lower AM colonization than oats treated with Evergol Energy (P < 0.05) (the control and Stamina were intermediate). Seed treatment didn’t affect biomass or P concentrations.
Saskatchewan Cropland Soil: Chickpea & Field Pea
A different study[ii] (Jin et al.) was conducted by U.Sask. soil scientists using local cropland soil. A single variety of chickpea and one of field pea were used. Chickpea treatments included Agrox FL (captan), Allegiance FL (metalaxyl), Apron Maxx RTA (fludioxonil & metalaxyl), Crown (carbathiin & thiabendazole), Trilex AL (trifloxystrobin & metalaxyl), or nothing. Field pea included Agrox, Allegiance, Apron Maxx RTA, Thiram 75WP (thiram), Vitaflo 280 (carbathiin & thiram), or nothing. Crops were planted in greenhouse pots in a 50:50 mix of the local cropland soil to sterilized sand (as you might guess, this improved drainage, which reduced disease pressure—the authors detected none; furthermore, all seeds were washed to sterilize the seed coat) and grown under warm conditions (70 – 80F). The local cropland soil had pH 7.5, and Olsen P of 20 ppm (moderately high; equivalent to 25 ppm Mehlich-3). Each of the six fungicide and the control treatments for each crop was further divided into some that were inoculated by a commercial single-species AM inoculant[iii] placed 0.4″ beneath the seeds, and some that were not. All seeds were supplied with high doses of the proper Rhizobial (legume) inoculant. Plants were harvested at eight wks.
For chickpea, Jin et al. found no statistical difference (P < 0.05) in percent of native AM colonization for any of the treatment’s vs control (Agrox stimulated AM colonization slightly, Crown was the same as control, the others slightly depressed it). Adding the AM commercial inoculant produced greater AM colonization in all treatments and exaggerated the differences (Agrox continued to stimulate AM slightly above control, though statistically not significant).
For field pea, there were no statistical differences in native AM colonization percent between the control, Thiram, and Agrox (Agrox again was slightly better than control, Thiram about the same); all the other treatments were significantly (P < 0.05) less colonized by AM, with Allegiance being the worst. When commercial AM inoculant was added, all the colonization percentages went up, but kept their overall relative ranking (Agrox > control = Thiram > Vitaflo > Apron Maxx > Allegiance). The authors point out that the contact-only fungicides Thiram, Agrox, and Trilex tended to be benign, and perhaps mildly beneficial—indeed Agrox boosted AM colonization by 4 – 5% (but changed the composition of the mycorrhizal community). Metalaxyl-containing products (Allegiance, Apron Maxx) looked more damaging, at least for these two legume crops (metalaxyl is systemic).
Using DNA sequencing, 39 species of AM fungi were detected in Jin et al., and all seed treatments profoundly affected the AM community composition on the roots for both chickpea and field pea. And yet no fungicide treatment significantly altered the total glomalin-related protein production in the soil. However, in field pea, biomass was sharply reduced (significant at P < 0.05) with Allegiance and Apron Maxx. In chickpea, biomass was significantly reduced by Trilex. The greatest biomass was the untreated controls for both crops, thus demonstrating the importance of AM fungi on these crops’ growth (remember, this is with seed coats being sterilized, and very benign growing conditions—well drained and quite a lot warmer than what these crop seeds typically experience for stand establishment in the field).
This study (Jin et al.) found that the single-species AMF inoculation produced 12 – 46% greater glomalin-related protein production in field pea soil, and 33 – 74% more in chickpea soil. However, Jin et al. conclude that this might only happen if the inoculum is placed away from the fungicide-treated seed, such as with a side-band opener. Furthermore, Jill Clapperton, soil ecology consultant (and previously, a scientist with Agri-Food Canada), cautions that AM inoculants placed onto the seed may not be a good idea anyway, since overwhelming the young seedling right away can be too taxing for it—yes, indeed, AM fungi in excess can cause yield drag! (So can Rhizobial inoculants applied too heavily to the seed—Clapperton prefers granular inoculants.) The plant controls AM colonization to a large degree, but in some cases, it takes on too much too soon in its life when inoculated at artificially high levels.
Other Studies
Cameron et al. discuss other studies, noting that a previous field study[iv] in Brazil by another group of scientists found a 46% reduction in AM levels on no-till corn (maize) grown from seed treated with fludioxonil (Maxim, a contact fungicide, which is a very common ingredient in seed treatments, including many of the flavors of Cruiser & Vibrance). Clapperton comments that tropical AM fungi aren’t nearly as robust asGlomus spp, which dominate cool-season prairies and other temperate ecosystems—she says the wimpiest of the tropical AM fungi only occur in places like Brazil and Australia and are strongly suppressed by N fertilization. Clapperton further comments that the % colonization in this study was very low overall.
A different field study[v] (Murillo-Williams & Pederson) of soybean seed treated with fludioxonil and/or mefenoxam (an isomer of metalaxyl, also sold as Allegiance, etc.) found “fludioxonil favored [emphasis added] AM colonization in nonfumigated soil, where fludioxonil-treated plants had double the root colonization of the control (6 vs. 2.8%, respectively) and five times more root colonization than plants treated with mefenoxam (6 vs. 1.1%, respectively). . . . No differences in grain yield, final stand, or grain composition were found among seed-applied fungicides or between nonfumigated and fumigated soil.” Jin et al. describe the effect of seed-applied fungicide actually boosting AM colonization as being caused by the displacement of aggressive pathogenic Rhizoctonia spp on the roots, as these compete for space and root resources.
In the study with which I opened this article (Cameron et al.), metalaxyl is an ingredient in all the oats treatments, as well as Evergol Energy SB for soybean, and no untoward effects were observed. Another study[vi] (considerably smaller) failed to find any effect from Raxil XT (metalaxyl + tebuconazole) on mycorrhizal levels on either corn, tomatoes, or zucchini in the greenhouse, although that study used only a single strain of arbuscular mycorrhizae (one sampling date for corn showed decreased AM colonization from captan, however). But two negative findings (Jin et al., plus Murillo-Williams) are enough to raise my eyebrows as to metalaxyl/mefenoxam. However, Tim Paulitz, USDA-ARS plant pathology scientist, says, “Metalaxyl—and mefenoxam, an isomer of metalaxyl—is specific for Oomycetes such as Pythium and Phytophthora. These are not true fungi, and these chemicals have little activity against true fungi [such as AM].” So perhaps those worrisome findings are flukes that will not endure when more evidence is compiled. Then again, maybe there’s a mechanism we don’t understand. Anyway, it’s enough to give me pause, and not want to use this chemistry without good reason (and there usually is—we’ll come back to this in a moment).
Both Cameron et al. and Jin et al. interpret the prior research as well as their own data as strongly supporting the conclusion that the effects of fungicides on AM are highly diverse, depending on which AM species, which fungicide, and which crop is studied. In their concluding remarks, Cameron et al. state that mycorrhizal diversity may be important in overcoming any negative effects from seed-applied fungicides. (Luckily, this is exactly what we have in long-term no-till fields with considerable plant diversity.)
Clapperton, in unpublished research, found that “fungicide seed treatments delay the initial colonization of the root by mycorrhizal fungi in the soil compared with seeds that were not treated. This delay was generally between one and two weeks after emergence. At V6 in corn, all the plants had the same percent of the root colonized by mycorrhizal fungi.”
Practical Aspects
Of all these studies, Cameron et al. is one of the most comprehensive for field crops, using three crop types, and a variety of common seed treatment ingredients (including fludioxonil & metalaxyl) with different characteristics. Furthermore, it uses four species of arbuscular mycorrhizae that are prevalent in South Dakota cropland (granted, there are dozens more that exist in SD cropland, but the scientists used the most prevalent four). They found little or no detrimental effects on the mycorrhizae, and occasional slight benefits (perhaps because root biomass or root health was improved). Jin et al. is also comprehensive for the two crops it covers, and while the contact fungicides look entirely benign or even beneficial to AM fungi and glomalin production, it’s slightly more disconcerting for some (not all) of the systemic ones—particularly for metalaxyl-containing seed treatments on field pea. The other studies are not particularly alarming, except for the one finding 46% AM colonization reduction in Brazilian no-till corn grown in the field from seed treated with fludioxonil—although that study looked at only one crop, one year, and only one hybrid (otherwise, their methodology seems solid enough; and they took theirs to yield, with the no fungicide no-till yielding 2.2% more than with fungicide, which was significant at P < 0.01). Why the different outcomes? Mainly because the species of AM weren’t the same between Brazilian and S. Dakota (or Sask) cropland, and the soil characteristics were markedly different. What is so weird about the Brazilian study is that fludioxonil isn’t even systemic in the plant! [vii] Not to mention that Murillo-Williams & Pederson found AM colonization to be improved two-fold by fludioxonil in soybean as compared to control. (And Cameron et al. found no effect from fludioxonil in either soybean or corn.) So, most likely the Brazilian study is a fluke, and the results wouldn’t survive attempts to replicate it even at that field site. Either way, it’s largely irrelevant to temperate cropping systems because those are dominated by Glomus [a robust group of AM fungi species] whereas tropical ones are not.
Paulitz offers this perspective: “Many seed treatments are transported up into the developing seedling, shoot, etc. [xylem-transport], but do give limited protection around the seed from concentrations diffusing into the soil surrounding the seed. But none I know of are true phloem-transported, i.e., move into the root tips. Certainly, in a more mature plant [i.e., not seedling stage], the roots away from the seed are probably not encountering very high concentrations of the chemical. And of course, it is the roots that AM fungi infect. Also, the fact that seed treatment chemicals are diluted out over time, either because they diffuse or wash away, or the roots leave that protected zone.”
In general, I fail to see much evidence for the crusade to eliminate seed-applied fungicides for the sake of mycorrhizae—you can pick out a study that either creates alarm or quells it, but taking them all together, there’s nearly as much evidence for improving AM colonization versus curbing it (which means it sort of all clusters around negligible to mildly negative effect, but perhaps even beneficial for AM colonization for some contact-only fungicides on the seed). There are other factors that typically have a much greater influence, such as crop type & genetics, the P level of the soil (high P suppresses AM colonization, but not entirely), and how much total plant diversity you have in your rotation (including cover crops, although brassicas are poor hosts for most species of AM fungi). And I’m assuming you’re not doing anything silly such as tillage—that really kills off the mycorrhizae (long chem-fallow periods aren’t good either).
Do you need seed-applied fungicides? It depends. Obviously, ‘organic’ growers get along without them, and everyone did prior to their invention. However, pressure from seedling diseases is often higher in no-till (because the seedbed is cooler and considerably wetter), especially in short rotations. Most studies[viii] show a decent ROI for wheat or barley treated with fungicides on the seed (indeed, if you have serious Pythium damping-off problems, metalaxyl/mefenoxam may be the difference between having a crop or not; also, loose smut gets pretty bad on barley without seed treatments—I’ve seen 30% losses). Evidence suggests a good ROI for corn, sorghum, and cotton too. I might be willing to go without fungicide on soybeans if seed quality is good and the no-till farmer isn’t planting unusually early.
Seed-applied fungicides get the crop off to a good start and often prevent later problems incurred by a weak crop and thus requiring large-scale interventions to try to mitigate. I would include replanting and/or thin stands in this—sometimes seed treatments are the difference between success and failure getting a stand in adverse conditions or with subpar seed lots. [ix] (Along with high-vigor seed, see my previous newsletter—note that high seed vigor is far more important than seed-applied fungicides.) And subpar stands certainly aren’t helping your field ecology anyway, as you’ll have more sunlight baking the soil, more raindrop impact, less mulch cover for future years, and fewer plants for mycorrhizae to colonize (slightly impaired AM colonization on a full stand will often be more tons/a of AM hyphae and glomalin than full colonization of a 90% stand). And probably fewer dollars in your bank account if the stand is reduced or more variable or the plants feebler. Not to mention that replanting really wrecks your mulch cover, even with ultra-low disturbance seeding tools. If everyone planted into ideal conditions (and it didn’t rain excessively afterwards or get cold) with exceptionally high-vigor seed that’s relatively free of seed-borne pathogens, we probably wouldn’t see any ROI from seed-applied fungicides. But it’s tough to fulfill all those criteria in the real world on large acreages, so we rely on seed treatments to mitigate the downside and make stand establishment more predictable (which lets us dial-in seeding rates more accurately).
Clapperton has these wise words for us: “I’m not all that concerned about [fungicidal] seed treatments. Do we need them everywhere? Probably not. Can we go without them entirely? I’m not on board with that.” Clapperton cautions against using fungicide seed-treatments with abandon, as there’s always consequences (some of which we don’t know), but says that, in general, the risk/benefit usually comes out in favor of using them, especially when soils are cold (for that crop species) or seed vigor is subpar, or when soils are wet or compacted (which also worsens seedling diseases). She says to keep it all in perspective, “Growing canola is a lot worse for mycorrhizae than fungicides, and we still grow canola! But the [rotational] system works because legumes are so strongly mycorrhizal and make up some of the deficit.” (Western Canada and PNW rotations often are canola >>cereal >>legume (“pulse”) >>cereal.)
And, to improve things further, Clapperton is a big fan of polyculture or intercropping or companion cropping, which is gaining popularity in that region (growing 2 grain crops simultaneously, usually on alternate rows—often these are harvested together, and the grains separated later; it can also mean having a cover crop growing in amongst the grain crop). Under this system, more strongly mycorrhizal plants can be growing at the same time as low-mycorrhizal plants, to keep AM fungal numbers high. (Modern small grains are not nearly as mycorrhizal as their wild ancestors; also, desi/wild-type chickpeas are much more mycorrhizal than kabulis.)
Returning to fungicidal seed treatments, Clapperton says that the active ingredient(s) aren’t doing all the heavy lifting—she says that nearly all the commercial formulations have micronutrients in them, often deliberately below the threshold to which they’d need to be listed on the label (and sometimes they are— Vitavax M has always had molybdenum in it). Clapperton says that having an adequate supply of essential heavy metals (Zn, Cu, Mn) is crucial for the plant to mount a robust immune defense against invading pathogens, and that ‘pickling’ these onto the seed is a great first line of defense.
Summarizing
Given the current evidence, for temperate climates, I’m not the least bit inclined to eliminate usage of fungicide seed treatments—except perhaps in soybeans when seed quality is good, and rotations not too short, and with well-drained soils. I might be extra cautious with seed-applied fungicides on tropical soils, however. (I know some of the low-input Regen Ag folks are saying they get along fine without any seed treatments whatsoever, but how accurately are they measuring their stands and yields? Did they even do any trials, or is this just a seat-of-the-pants, yeah, I-got-a-stand type of affirmation? I know about the Precautionary Principle of not wanting to risk damaging your soil ecology—and if you take that Principle very far, you wouldn’t get out bed in the morning! And I will add that you can have the healthiest soil in the world and still go broke farming.)
The amount of soil volume being affected by normal rates of fungicidal seed treatments tends to be small enough that AM aren’t usually seriously inhibited after a couple weeks. And with some careful selection of which fungicide active ingredients, you use on the seeds (perhaps avoiding metalaxyl/mefenoxam, although doing so greatly increases your risk of Pythium stand losses[x]), even that small risk can largely be eliminated. If you really want to improve your AM colonization, more worthwhile efforts are to grow more stuff, include more diversity of plants (especially highly mycorrhizal species and varieties), intercropping, don’t over-supply P, and eliminate soil disturbance. In corn, for instance, GMO hybrids tend to be less mycorrhizal on average (according to Clapperton), so don’t plant them without good reason (however, some GMO hybrids are actually more mycorrhizal). (By the way, you can also increase mycorrhizae in your soil by not killing the weeds—but I don’t recommend taking that approach very far.)
[i] J.C. Cameron, R.M. Lehman, P. Sexton, S.L. Osborne & W.I. Taheri, 2017, Fungicidal Seed Coatings Exert Minor Effects on Arbuscular Mycorrhizal Fungi and Plant Nutrient Content, Agron. J. 109: 1005–1012.
[ii] H. Jin, J.J. Germida, F.L. Walley, 2013, Suppressive effects of seed-applied fungicides on arbuscular mycorrhizal fungi (AMF) differ with fungicide mode of action and AMF species, Appl. Soil Ecol. 72: 22– 30.
[iii] ‘Mycorrhizae ASP’ by Premier Tech Biotech, Quebec, containing Rhizophagus irregularis, formerly Glomus irregulare.
[iv] M. Castelli, R.C. Urcoviche, R.M.T. Gimenes & O. Alberton, 2014, Arbuscular mycorrhizal fungi diversity in maize under different soil managements and seed treatment with fungicide, J. Food Agric. Environ. 12: 486–491 (it may or may not be relevant, but the rate of fludioxonil a.i. used in this study was 50% higher than the labelled rate in USA for Maxim 4FS—they applied 150 mL of 25 g/L material onto 100 kg of seed.)
[v] A. Murillo-Williams & P. Pederson, 2008, Arbuscular Mycorrhizal Colonization Response to Three Seed-Applied Fungicides, Agron. J. 100: 795-800.
[vi] R.L. Burrows & I. Ahmed, 2007, Fungicide seed treatments minimally affect arbuscular-mycorrhizal fungal (AMF) colonization of selected vegetable crops. J. Biol. Sci. 7:417–420.
[vii] C.J.R. Klittich, F.R. Green III, J.M. Ruiz, T. Weglarz & B.A. Blakeslee, 2008, Assessment of fungicide systemicity in wheat using LC-MS/MS, Pest Mmgt. Sci. 64: 1267–1277. See also https://www.extension.purdue.edu/extmedia/BP/BP-70-W.pdf. And Maxim literature describing it as only a contact fungicide.
[viii] For example, Evergol Energy provided a 2.1 bu/a yield response in small grains over a 5-yr period across 5 northern states; wheat yields were low 40s on average, so a 5% yield response vs untreated across all tillage systems. (Source: Bayer Powerpoint.) A study in the PNW found 3 – 10% yield response in winter wheat for common seed treatments (various Raxil and Dividend formulations); the PNW has rather intense disease pressure due to short rotations. (R.J. Cook, D.M. Weller, A. Youssef El-Banna, D. Vakoch & H. Zhang, 2002, Yield responses of direct-seeded wheat to rhizobacteria and fungicide seed treatments. Plant Dis. 86: 780-784.) Across 26 environments during 3 years in western Canada, seed fungicide + insecticide treatments provided an excellent ROI in winter wheat at a lower seeding rate of 200 seeds/m2 (~800,000 seeds/a), but not at a heavier seeding rate of 400 seeds/m2. (B.L. Beres, T.K. Turkington, H.R. Kutcher, B. Irvine, E.N. Johnson, J.T. O’Donovan, K.N. Harker, C.B. Holzapfel, R. Mohr, G. Peng & D.M. Spaner, 2016, Winter Wheat Cropping System Response to Seed Treatments, Seed Size, and Sowing Density, Agron. J. 108: 1101-1111.) In a cotton study, “Plant stand was significantly greater in the Apron XL + Maxim 4FS + Systhane 40WP + Dynasty CST seed treatment as compared to the untreated control at 43 DAP. Plant stands were low with 26 to 13 percent seedlings surviving . . . Seed cotton yields were significantly increased by all fungicides that increased plant stand,” with yield increases up to 19.8% observed (the same treatment that had significantly better stand). (K.S. Lawrence, S.R. Moore & B.E. Norris, 2012, Evaluation of Seed Treatment Fungicides for Seedling Disease Management in North Alabama, 2011, in AU Crops: Cotton Research Report: March 2012 [Alabama Agricultural Experiment Station: Auburn Univ.].)
[ix] Seed-applied fungicides can actually improve germination scores of weak seedlots, although they will never be high-vigor. In 2018, most of the soybean-growing areas of the USA & Canada were plagued with excessive rain on mature soybeans before they could be harvested, resulting in low-vigor seed for 2019. Without seed-applied fungicides on the weaker seed lots, there would be a lot of disastrously poor stands (with no seed to replant), or acres would need to be diverted to other crops for lack of seed. This happens once every decade or so. Sorghum seed production sometimes has similar issues.
[x] There’s an alternative for Pythium control—ethaboxam, marketed as Intego Solo by Valent and labelled for cereals, corn, etc. However, we don’t have any evidence as to whether it’s any less risk to AM fungi or not. One university researcher comment, “Ethaboxam is a thiazole carboxamide fungicide (group 22) and [I] suspect that it could adversely impact AMF fungi but am not aware of any literature in this area.”
